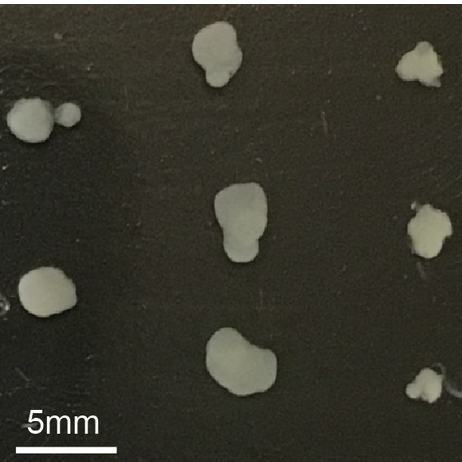
Like many around the world, the lab of Professor Mriganka Sur in The Picower Institute for Learning and Memory at MIT has embraced the young technology of cerebral organoids, or “minibrains,” for studying human brain development in health and disease. By making a surprising finding about a common practice in the process of growing the complex tissue cultures, the lab has produced both new guidance that can make the technology better, and also new insight into the important roles a prevalent enzyme takes in natural brain development.
To make organoids, scientists take skin cells from a donor, induce them to become stem cells and then culture those in a bioreactor, guiding their development with the addition of growth factors and other chemicals. Over the course of weeks, the stem cells become progenitor cells that multiply and then go on to become, or “differentiate” into, neurons or other brain cell types. Along the way the cells also migrate within the growing blob to take their places forming basic brain structures and circuits.
A beauty of organoids, therefore, is that as the cells in the culture grow and develop together, they simulate many of the basic processes that occur when real brains take shape. When cell donors have genes that cause disease, the organoid grown from their cells will reproduce underlying disease characteristics. The Sur lab uses organoids to study the abnormal brain development in Rett Syndrome, a devastating autism-like condition with a genetic underpinning.
A common practice among labs growing organoids is to improve the viability of the cells by adding a small molecule chemical called CHIR 99021 to inhibit the activity of a ubiquitous natural enzyme called GSK3-beta. In the new study in PLOS ONE the team led by Picower Fellow Chloé Delépine, confirmed that while different doses of CHIR 99021 can indeed keep cells alive, they have opposite effects on organoid growth – low doses promote growth but high doses constrain it and very high doses will stop it altogether. That information alone has obvious implications for labs using varying doses of CHIR 99021, but because Delépine’s team investigated how these growth effects occur, it also helps to clarify what GSK3-beta activity affects during brain development.
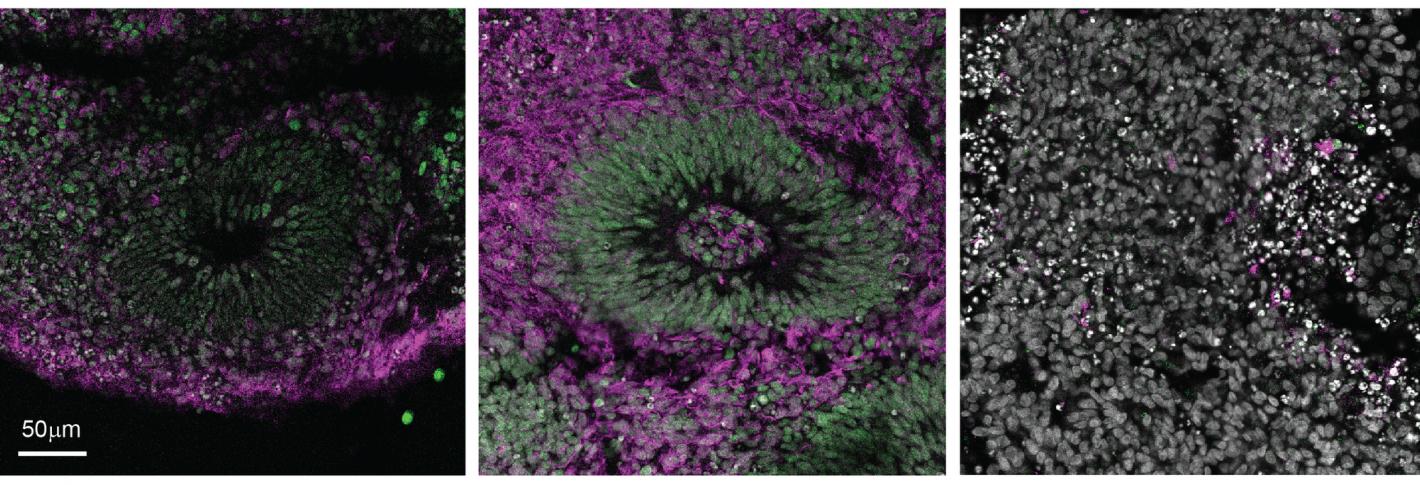
Above: Researchers tracked molecular markers of neural progenitor cells in organoids including one called PAX6, in green. Note that the middle organoid, which received a low dose of CHIR 99021, has more green staining and therefore more neural progenitor cells, than either the control organoid on the left or the high-dose organoid on the right.
It’s an important question. Other research groups, for instance, have shown that perturbations in a signaling pathway involving GSK3-beta in the brain are associated with schizophrenia and autism. The new findings model how varying levels of inhibition may affect development.
“It’s is not just increasing the viability of the cells, it is also changing other cellular processes such as division, differentiation and migration,” Delépine said. “Our idea was to test the effects of different doses and to better understand these mechanisms we observed.”
Different doses, opposite effects
To conduct the study, Delépine’s team cultured organoids and, from day 15 through 35 of their development, treated them with doses of either an inert control, or 1, 10 or 50 micromolar of CHIR 99021. They then tracked various properties of cells by staining for different molecular markers of those properties.
Straight away, she noticed major differences in organoid size depending on the dose received. Low dose (1 micromolar) organoids were 1.6 times bigger than controls, but high dose (10 micromolar) organoids were 1.8 times smaller and very high dose (50 micromolar) organoids simply stopped growing at all early into treatment.
The growth differences were not because of cell death. As expected given that labs use CHIR 99021 to improve cell survival, adding CHIR 99021 enhanced cell viability and the effect was stronger for high dose than low dose treatments. But when the lab stained for markers of proliferation, or cell division, they found that high-dose organoids exhibited less while low-dose organoids exhibited more than controls.
When the team looked at another cellular activity that could affect growth they found a similar pattern. In high-dose organoids fewer cells differentiated into neural progenitor cells needed to produce new neurons while in low-dose organoids proliferation increased. As a result, high-dose organoids had fewer neurons.
Additionally, the low dose of CHIR 99021 resulted in more migration of newborn neurons to places needed for structural development of the organoid than control treatment did, suggesting that slight inhibition of GSK3 beta contributes to increased migration.
Delépine noted that the growth-promoting effects of low doses or the growth-limiting effects of high doses are not “good” or “bad.” They are just levels of control of the culture. The new study provides more insight into how to use CHIR 99021 to achieve the desired end.
“It really depends on the goal of the organoid research that you are doing,” she said. “Depending on what you want, different doses of this molecule can help you achieve a different phenotype.”
And in natural brains, the study suggests, GSK3-beta likely plays a key role in the proliferation of progenitor cells, their differentiation into mature brain cells, and the propensity of those cells to migrate.
In addition to Delépine and Sur, the paper’s other authors are Vincent Pham and Hayley Tsang.
The National Institutes of Health, The Simons Foundation Autism Research Initiative and the JPB Foundation supported the research.