For many Picower Institute neuroscientists, asking some of their most important research questions requires pushing the limits of live imaging technology. It takes vision to see the brain in action.
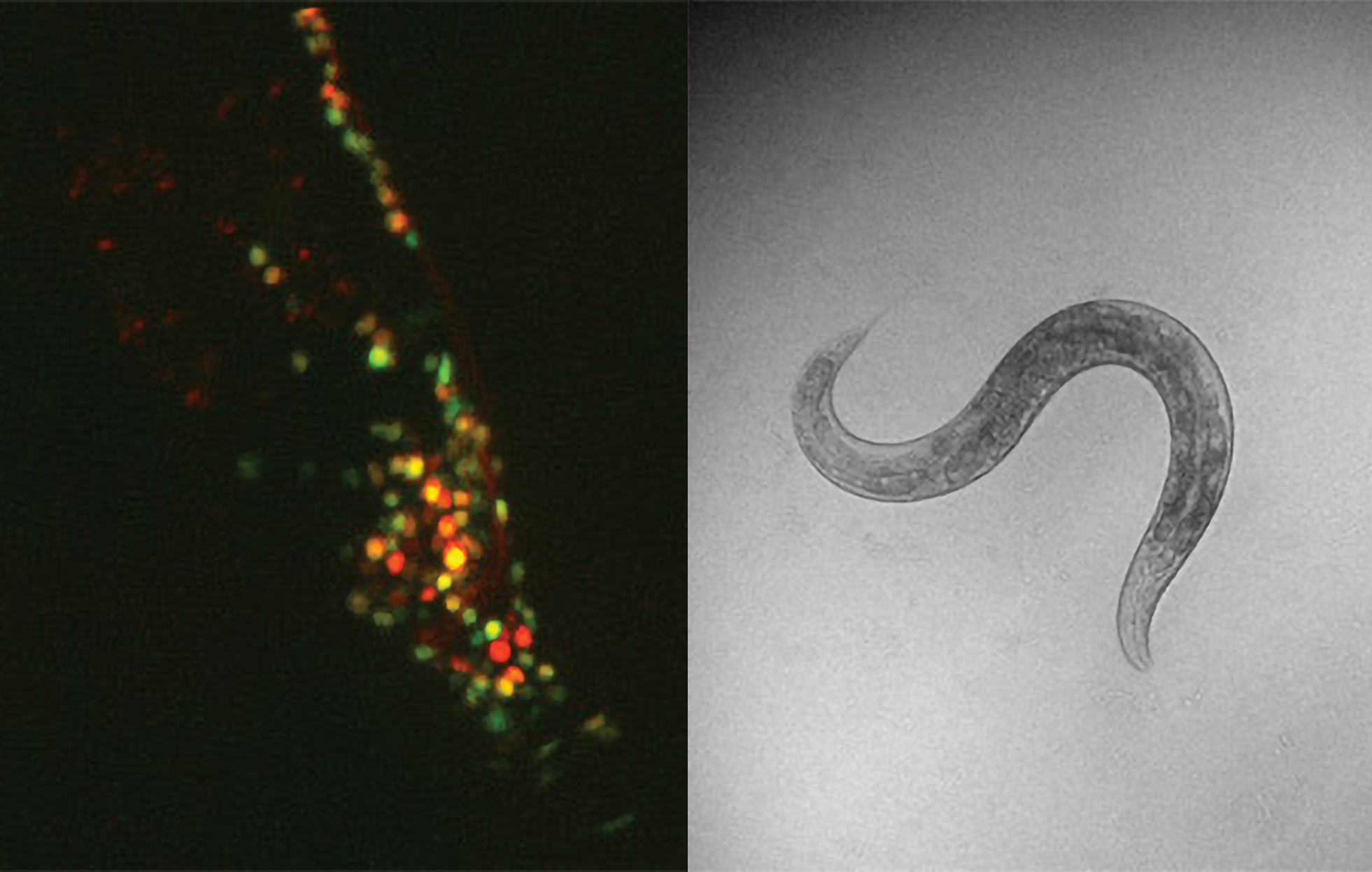
Back in 2016 as Associate Professor Steve Flavell imagined how his new lab would study how whole nervous systems produce sustained but flexible behaviors, he envisaged a microscope capable of tracking every behavior and the activity of every neuron in the simple brain of his model organism, the C. elegans worm. It took almost a decade of ingenuity, building on the previous inventions of other neuroscientists, but now Flavell’s lab has begun making striking discoveries.
“For a long time it was a dream of neuroscientists to be able to make all of these measurements to see everything,” Flavell said. “Because how can you relate neural activity and behaviors if you can’t record them all at the same time?”
Similarly inspired, neuroscientists across The Picower Institute are advancing live imaging techniques to reveal fundamental mechanisms of memory, how animals learn to intelligently navigate their environments, and how they decide to act on perceptual cues. Meanwhile, others are developing different live imaging techniques to discover how the brain forms and remodels the circuits that enable these essential functions.
The appeal of watching the brain work seems intuitive, but little of what’s happening can be seen directly. Neural communication activity (i.e. when neurons electrically “spike” to transmit signals) is electrochemical, so it’s invisible. Electrodes can directly tap these signals (a practice called “electrophysiology”), but for some studies, it’s not feasible to precisely poke many wires into the brain of a living, moving animal. Moreover, electrodes can’t reveal which exact neurons they are eavesdropping on.
While the brain’s anatomy is visible, it requires great cleverness to see it in ways that answer many crucial neuroscience questions. Sometimes it’s enough to put a stained slice of tissue under a microscope to capture a fixed, single moment in time. But Picower scientists seek to observe how these structures change daily in animals as they develop or see and learn new things.
Meanwhile, live imaging techniques suited for human volunteers, such as MRI, can’t resolve the scale of molecules, cells and circuits that many Picower Institute researchers must see to understand fundamental mechanisms of brain function. The methods to use depend on what a scientist wants to know. Some Picower labs don’t emphasize live imaging, but those that do have surged to the vanguard.
Tracking activity and behavior
Flavell’s dream to image all behaviors and the whole brain activity of llive, freely moving animals required two revolutions. One was a way to make the brain’s electrical activity visible. That arose when neuroscientists engineered neurons with a protein that glows when calcium ions build up (a correlate of spiking). The other was the advent of artificial intelligence powerful enough to automatically track worm movement and behavior and also to spot the glowing cells in images.
When Flavell arrived at MIT, like-minded scientists at Princeton and Harvard were starting to develop prototype microscopes to image behavior and simultaneous neural activity. The first postdoc Flavell hired, Ni Ji, came from the Harvard lab. Ji and Flavell had their first version of a system up and running within a year, but even so, imaging defined cells across the entire brain of the worm along with its full repertoire of behaviors remained beyond any lab’s reach. For a study examining how worms integrate sensory information to decide on the optimal feeding behaviors, for instance, Ji imaged a small selection of the hundreds of neurons in the worm’s brain.
Lab members iteratively improved the system, including its ability to follow the worm’s movements to keep it perfectly centered. But the breakthrough needed for whole-brain imaging emerged when two graduate students who arrived in 2018, Adam Atanas and Jungsoo Kim, were home during the pandemic. They wrote software that ensured that no matter how much the worm wriggled and twisted, thereby shifting, warping and sometimes partially obscuring the neurons within them, the computers could still keep track of the cells to register their flashes of activity.
Last year saw big payoffs from those efforts. The lab published two studies in Cell that employed whole-brain imaging. In one they unveiled a predictive model of how most of the worm’s neurons encode its behaviors. In the other they mapped out how the worm’s whole nervous system responds to the neuromodulatory chemical serotonin, which in humans is the most frequent target of psychiatric drugs.
Deeper insight
For studies of cognitive abilities such as how mice learn to act on perceptual cues, Newton Professor Mriganka Sur is also a calcium imaging “power user.” But mice present different challenges than worms. Mouse brains have millions of neurons and are thicker and opaque. Calcium imaging requires external light stimulation, which has trouble penetrating the tissue. So-called “2-photon” microscopes can image calcium flashes a little way below the brain's surface, but Sur’s lab wanted to visualize activity through the brain’s entire cortex, which is where mice (and humans) perform sophisticated information processing. About eight years ago Sur therefore teamed up with former postdoc Murat Yildirim and Mechanical Engineering Professor Peter So to refine the nascent concept of “3-photon” microscopy (first developed at Cornell). In 2019 they published the first study to image live neural activity all the way through the cortex’s six layers, a depth of more than a millimeter.
Sur’s lab has also pioneered calcium imaging of targeted neuronal populations that connect disparate regions, for example the prefrontal cortex and the superior colliculus. Alongside, they have imaged the activity of single axons that connect brain regions to decipher the communications such pathways enable. And they have developed tools to simultaneously image multiple cell types, such as neurons and astrocytes, to understand how they influence each other within cortical networks.
Sur has also tackled calcium imaging’s slowness. Calcium flashes last hundreds of milliseconds but a single spike is comparatively instantaneous. When spikes occur rapidly, calcium imaging fails to resolve each cleanly. To improve inference of spiking patterns from calcium signals, Sur has co-authored two studies, including one last year, presenting increasingly efficient algorithms.
A ‘new frontier of imaging’
In parallel, scientists are developing a quicker technology to visualize neural electrical activity, called Genetically Encoded Voltage Indicators, or GEVIs. Among them are new Picower Institute Assistant Professor Linlin Fan, and Y. Eva Tan Professor of Neurotechnology Ed Boyden, an affiliate member of The Picower Institute. GEVIs are becoming so quick and sensitive that their glow can indicate a neuron’s voltage even if it’s less than the peak represented by a spike. GEVIs therefore offer the potential to view subtle neural activity across wide areas of an animal’s brain with enough resolution to show activity in individual cells and enough quickness and sensitivity to rival electrophysiology.
Though it’s still emerging, that promise intrigues Sherman Fairchild Professor Matt Wilson, whose decades of electrophysiology innovations have advanced studies of how rodents learn and remember how to navigate their physical environments. The work requires tracking activity among hundreds of neurons in multiple brain regions. Recently Wilson and postdoc Jie “Jack” Zhang began collaborating with Boyden and his team to develop a system that optimizes a GEVI, a new microscope design, and a new kind of camera.
“This is pursuing the technology that will allow us to use the same principles that we’ve embraced—direct recording of electrophysiological activity—and move that into the new frontier of imaging,” Wilson said. “You want to be able to ask and address questions and not be limited by the technology.”
Their first testbeds are larval zebrafish, which are transparent and have simpler brains than mice but more complex ones than C. elegans. The goal is to image brainwide with enough resolution and speed to capture meaningful neuroscience measurements. A key limitation to overcome, Wilson said, is extracting as much signal as possible. When GEVIs are used in mice, the light they emit will be dampened by the brain’s opacity. That’s where the novel camera comes in. It’s designed so that each pixel in the sensor can be individually controlled. The collaboration’s strategy is to control neighboring pairs of pixels so that in the same spot one pixel will image quickly (to capture fast neural dynamics) while the other stays on longer (to gather more total light).
The work is progressing well, Wilson said, with two papers on their systems currently under review by journals.
Although in a spatially focused way, rather than brain-wide, Fan is already applying GEVIs in mice and doing so in combination with another technology, optogenetics, which enables neural activity to be controlled with light. As a graduate student at Harvard and then a postdoc at Stanford she led papers in Cell in which she pioneered the combination of these methods to produce new insights into how circuits in the cortex processes the sense of feel and how neural activity in the hippocampus changes circuit connections to encode spatial memories.
These unique feats required more than just getting the GEVIs to work properly. With two paths of light going into the brain (one to stimulate the GEVIs and one for optogenetic control), and another coming out from the GEVIs, Fan’s team had to design microscopes that could avoid interference. Using a sophisticated arrangement of advanced optical components, she precisely sculpted the incoming light sources to target different parts of cells in very close proximity.
With a long-term goal of studying how the momentary neural activity upon encountering something new ultimately produces an enduring neural encoding of that memory, Fan is eager to push the limits of “all optical physiology” to enable investigations of larger populations of neurons in wider areas of the brain. Memories are encoded in circuits and their formation, storage and recall can depend on multiple brain regions.
“We are actively paving the way, by showing these are useful and functional in vivo,” Fan said. “We are one of the few groups really using these tools to discover new knowledge.”
To help advance the field’s techniques and their use, Fan is co-organizing a three-day conference in Paris in June: “Sculpted Light in the Brain.”
Structure & Physiology
Sculpting light to see and control activity is one thing. Using live imaging to reveal how the brain sculpts itself is another. That’s what has motivated other Picower Institute professors to make their own imaging innovations.
Like Flavell, William R. and Linda Young Professor Elly Nedivi knew she’d need new imaging technologies right from the establishment of her lab in 1998. She wanted to study the molecular basis of “plasticity,” the way the brain builds and edits circuit connections between neurons, called synapses, to enable learning and memory. Nedivi didn’t want to rely on making statistical inferences from observations in dissected brain slices—she wanted to directly watch it happen. At the time, one couldn’t buy a two-photon microscope to image in mice, but she engaged the help of So, setting off a collaboration that continues today.
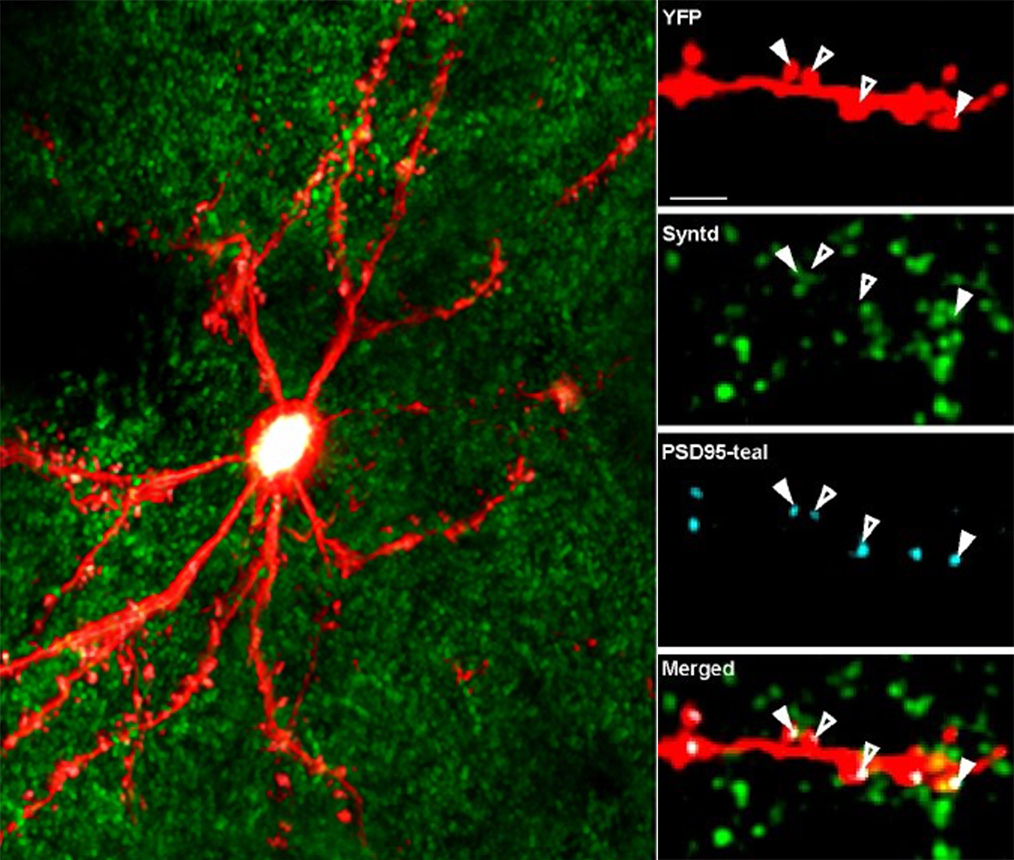
With that first scope Nedivi was able to see entire dendritic arbors (the vineline branches that extend from the neuron body) of individual cortical neurons and track them over time, seeing they were not as hardwired as everyone thought. The changes were subtle but one could track them by watching daily in the same animal. Inhibitory neurons constantly remodeled connections with their excitatory partners.
Inhibitory synaptic connections had never been visualized live before because they don’t form a distinguishable shape like the excitatory synapses that reside on small spine protrusions that are physically apparent. This led Nedivi to a new challenge: How can the two different kinds of synapses—excitatory and inhibitory— be tracked as they come and go, or shrink and grow? Doing that would require developing synaptic fluorescent markers and two-photon microscopy for simultaneous tracking of multiple colors, something she and So developed together.
First they developed a two-color system. Then, when Nedivi and So developed a three-color version, it meant she could image both excitatory and inhibitory synapses at the same time. That enabled her to discover that even when excitatory synapses were mostly stable in the adult brain, inhibitory ones would often come and go dynamically to modulate the degree of excitatory activity.
“We were only able to figure that out because we saw them right next to each other at the same time,” said Nedivi, who notes that many other labs have now adopted multi-color imaging.
Last year, the three-color system extended to also visualize specific inputs to synapses. This enabled her lab’s unprecedented analysis in Nature Neuroscience of how inputs from a brain region called the thalamus delivered sensory information to neurons in layer 2/3 of the visual cortex.
Now Nedivi, So, and graduate student Kendyll Burnell are developing a four-color system. As before, adding a color takes a lot of tinkering both with the microscope optics and with the proteins, or “fluorophores,” that glow to mark a key molecule. In this round, for instance, one of the new fluorophores turned out to be so bright that they had to ratchet down how much they stimulated it to avoid drowning out the others. Once four colors are fully working they hope to use it to examine how the thalamus connects to mature vs. immature synapses and to track differences in the synapses’ molecular compositions.
Menicon Professor Troy Littleton has developed new live imaging methods for his studies of how neurons in drosophila fruit flies develop their circuit communications infrastructure. In a 2018 study in eLife, his lab debuted “optical quantal imaging” in which they engineered synapses to flash whenever the neurotransmitter glutamate crossed from the sending cell’s side of the synapse to the receiving cell’s side. The technique yielded the insight that along the same neuron’s connection to a muscle a few synapses become very active while most remain comparatively weak. Then, using “intravital imaging” they were able to anesthetize, image, and then revive larval fruit flies to measure the day-by-day development of these different synapses. Last year, Littleton’s lab used intravital imaging again in a study showing that without the protein perlecan, neural axons can literally unravel, disrupting synapse formation.
And the Sur Lab’s three-photon microscope has provided deeper looks at structures thanks to another technology embedded in the scope called “Third Harmonic Generation.” THG detects differences in how materials bend light. It can therefore resolve the membranes of cells and blood vessels. In 2020 the lab used THG to examine how the functions of distinct brain regions correlated with differences in their structure. And in 2022 they used the scopes to image advanced 3D cell cultures modeling early brain development in Rett syndrome. They showed that newborn neurons in the cultures struggled to migrate to their proper places, lending insight into how disease symptoms develop.
Driven to discover, Picower Institute labs are advancing live brain imaging.