The complexity of understanding and treating Huntington’s disease, a fatal neurodegenerative disorder, is not in the cause. Instead what confounds scientists is how and when the well-known mutation of the Huntingtin gene sets off cascades of destruction deep within the brain. To better understand disease emergence and therefore find earlier targets for therapeutic intervention, MIT Associate Professor Myriam Heiman will use a major new award from the National Institutes of Health to use highly sensitive measures of gene expression early in the disease progression in mouse models.
In 2020, Heiman’s lab at The Picower Institute for Learning and Memory published two pioneering studies that revealed important potential mechanisms of Huntington’s disease progression, which especially punishes cells called spiny projection neurons (SPNs) in a brain region called for the striatum. One study revealed how certain genes that regulate the expression of others seemed crucial for promoting survival in the cells. The other investigation showed that a major problem is that RNA leaks out of the cells’ mitochondria, and becomes misinterpreted as an “invader,” setting off a destructive innate immune response.
But the studies, which were conducted in six-month-old mice, also raised new questions including when do such fateful cascades of immune activation and gene expression dysregulation begin, and what sets them off. Supported by a prestigious new eight-year Research Program Award (R35) grant from the National Institute for Neurological Disorders and Stroke, Heiman will probe for the roots of such problems, and seek to discover any new ones, in more and younger mice – at substantially earlier time points when pathology may be just beginning to develop.
“In mice we can look at the earliest stages of disease progression,” said Heiman, who is appointed in the Department of Brain and Cognitive Sciences. “Going earlier in phenotypic time and using very sensitive techniques can help us look at what’s initiating this cascade of negative events.”
In these younger mice the lab will use techniques such as TRAP to assess how DNA accessibility and gene transcription and translation vary with varying degrees of Huntingtin mutation in a wide variety of cell types – not only SPNs but also neurons that connect to them and supporting “glial” cells.
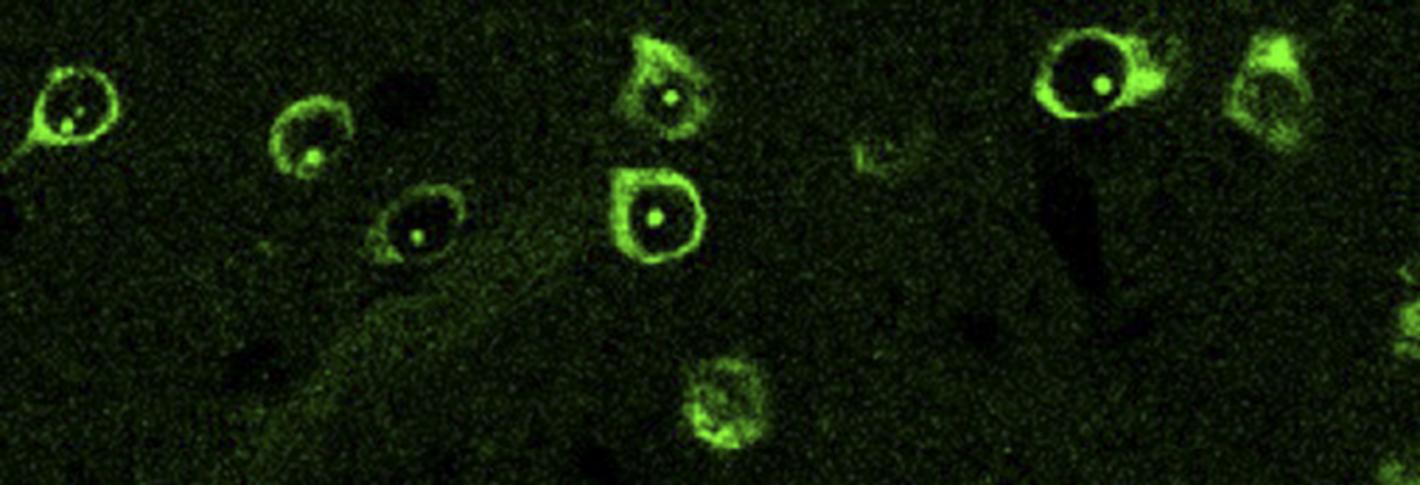
Above: Spiny projection neurons labeled for analysis with TRAP technology. Image courtesy Heiman Lab.
As they identify targets that appear to be potential culprits in fomenting pathology, they will then validate their likely significance by cross-checking them against measurements made in early stage post-mortem human tissues. The most promising candidate “driver genes” can then be systematically knocked down in mice to observe the physiological and behavioral effects of their loss.
Similarly, the team will look for potential early triggers of the mitochondrial RNA release and subsequent destructive immune response by perturbing genes with a suspected role.
While Heiman has proposed a specific plan for the research, the nature of the prestigious R35 award is to support the researcher as much as the research. That means she has the flexibility to change directions and pursue new leads that may emerge from the large amount of new data her lab’s experiments will produce.
“I’m truly honored to have received this award,” Heiman said. “I’m delighted that this will broadly support our Huntington’s disease work going forward.”





