When patients undergo general anesthesia, their brain activity often slows down as they sink into unconsciousness. Higher doses of anesthetic drugs can induce an even deeper state of unconsciousness known as burst suppression, which is associated with cognitive impairments after the patient wakes up.
A new study from MIT, in which the researchers analyzed the EEG patterns of patients under anesthesia, has revealed brain wave signatures that could help anesthesiologists determine when patients are transitioning into that deeper state of unconsciousness. This could enable them to prevent patients from falling into that state, reducing the risk of postoperative brain dysfunction.
One of these distinctive patterns emerged in the brain’s alpha waves (which have a frequency of eight to 14 cycles per second). Once patients became unconscious, these waves started to wax and wane in amplitude. As patients went deeper into unconsciousness, the pattern of this waxing and waning in amplitude, or amplitude modulation, continually changed.
“If you track this modulation as it gets deeper or shallower, you have a very principled way to track level of unconsciousness under anesthesia,” says Emery N. Brown, the Edward Hood Taplin Professor of Medical Engineering and Computational Neuroscience and a member of MIT’s Picower Institute for Learning and Memory and the Institute for Medical Engineering and Science.
Brown, who is also part of MIT's Aging Brain Initiative, is the senior author of the new study, which appears this week in the Proceedings of the National Academy of Sciences. The lead authors of the paper are Picower Institute research scientist Elie Adam, Ohyoon Kwon ’20, and graduate student Karla Montejo.
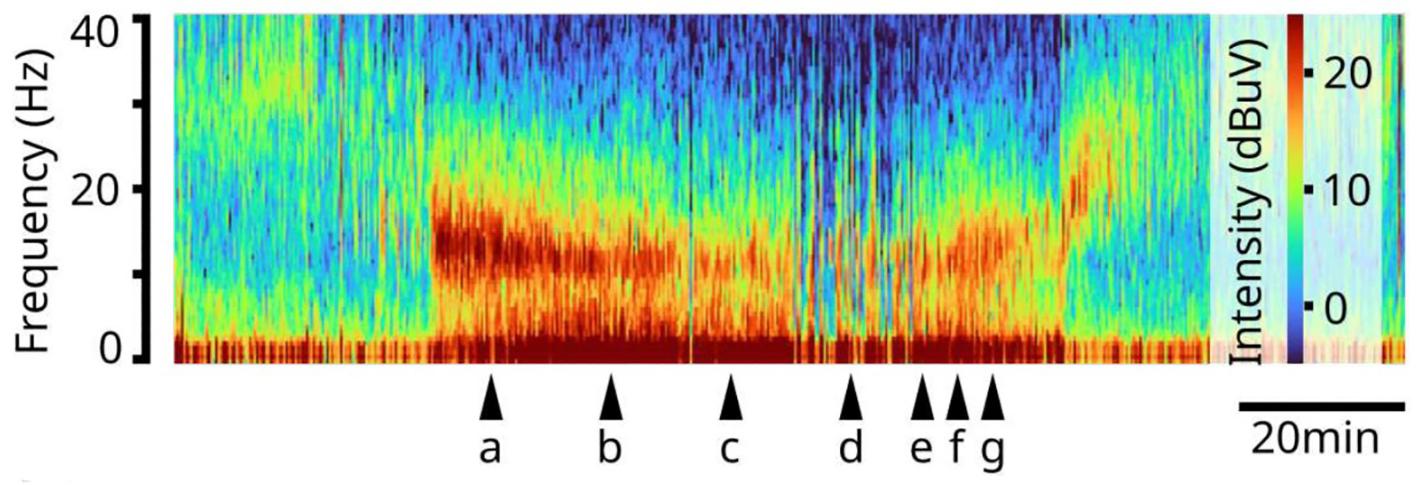
Above: A spectrogram of EEG readings from a study volunteer under propofol general anesthesia. Brain rhythm frequency is lower at the bottom and higher at the top. Warmer colors indicate greater intensity of rhythms. The patient loses consciousness before "a," reaches burst suppression at "d" and regains consciousness after "g"
Measuring brain waves
Brain waves, which are generated by synchronized neuronal activity, oscillate at different frequencies depending on what kind of task the brain is performing. When the brain is strongly engaged in mental activity, it produces higher-frequency beta (15-30 hertz) and gamma (greater than 30 hertz) oscillations, which are believed to help organize information and enhance communication between different brain regions.
Commonly used anesthesia drugs such as propofol have a significant effect on these oscillations. During anesthesia induced by propofol or other anesthetics that increase the effectiveness of GABAergic inhibitory receptors in the brain, the brain enters a state of unconsciousness known as slow-delta-alpha (SDA). This state is characterized by slow (0.1-1 hertz), delta (1-4 hertz) and alpha (8-14 hertz) oscillations.
With higher doses of these anesthetic drugs, the brain can fall into an even deeper state of unconsciousness. When in this state, known as burst suppression, EEG recordings from the brain show long periods of inactivity, punctuated by brief bursts of low-amplitude oscillations. When patients enter this state, they are more likely to experience postoperative confusion, delirium, and memory loss. These effects, which can last for hours, days, weeks, or months, are more common in elderly patients.
SDA and burst suppression produce distinctive EEG patterns that have been well-studied. However, they have been studied as separate brain states; what happens during the transition between the two states is less clear. That transition is what the MIT team set out to analyze in this study.
To do that, the researchers studied 10 healthy volunteers and 30 patients who were undergoing surgery. Most of the patients received propofol intravenously, and the rest received sevoflurane, a commonly used anesthetic gas. Both of these drugs act on GABA receptors in the brain, which reduce neuron excitability.
As the dosage of propofol was increased, patients showed two distinctive patterns of change in their EEGs. The first pattern was seen in the alpha waves, which started to wax and wane. As the dose increased, waxing was shortened and waning was prolonged, until the patient reached the state of burst suppression.
“You can see a very strong modulation, which is always there. As the modulation gets to be more profound, it eventually flattens out, and that's when the brain reaches the deeper state,” Brown says.
When the amount of drug was reduced, the amplitude of the alpha waves began to increase again.
The researchers also found a distinctive pattern in the slow and delta waves seen in the patients’ EEG readings. Slow and delta oscillations are the slowest brain waves, and as the amount of drug was increased, the frequency of these waves became slower and slower, reflecting a decrease in brain activity.
Metabolic disruption
The researchers hypothesize that propofol exerts these effects through its influence on neuron metabolism. The drug is postulated to disrupt the production of ATP, the molecules that cells use to store energy. As ATP production declines, neurons eventually become unable to fire, leading to burst suppression.
“This is consistent with the observation that burst suppression is very frequent in older patients, because their metabolic state may be less well-regulated than that of younger patients,” Brown says.
The findings could offer anesthesiologists more refined control over a patient’s state of unconsciousness during surgery, says Brown. He now hopes to develop an algorithm that could generate a warning that a patient is approaching burst suppression, which could be displayed on a monitor in the operating room. He says that anesthesiologists could also learn to make that determination by looking for these patterns in a patient’s EEG.
“One of the reasons we're excited about this is that it’s something you can actually see in the raw EEG,” Brown says. “Now that we have pointed out these patterns, they’re very easy to see.”
The researchers now plan to further explore what is happening to the brain’s metabolism during the transition to burst suppression, using animal models.
The research was funded in part by The JPB Foundation, The Picower Institute for Learnign and Memory, and the National Institutes of Health.
--From MIT News





