Using an innovative microscopy method, scientists at The Picower Institute for Learning and Memory at MIT observed how newborn neurons struggle to reach their proper places in advanced human brain tissue models of Rett syndrome, producing new insight into how developmental deficits observed in the brains of patients with the devastating disorder may emerge.
Rett syndrome, which is characterized by symptoms including severe intellectual disability and impaired social behavior, is caused by mutations in the gene MECP2. To gain new insight into how the mutation affects the early stages of human brain development, researchers in the lab Mriganka Sur, Newton Professor of Neuroscience in MIT’s Department of Brain and Cognitive Sciences, grew 3D cell cultures called cerebral organoids, or minibrains, using cells from people with MECP2 mutations and compared them to otherwise identical cultures without the mutations. Then the team led by postdoc Murat Yildirim examined the development of each type of minibrain using an advanced imaging technology called third harmonic generation (THG) three-photon microscopy.
THG, which Yildirim has helped to pioneer in Sur’s lab working with MIT mechanical engineering Professor Peter So, allows for very high-resolution imaging deep into live, intact tissues without having to add any chemicals to label cells. The new study, published in eLife, is the first to use THG to image organoids, leaving them virtually undisturbed, Yildirim said. Previous organoid imaging studies have required using technologies that cannot image all the way through the 3D tissue, or methods that require killing the cultures: either slicing them into thin sections or chemically clearing and labeling them.
Three-photon microscopy employs a laser, but Yildirim and So custom engineered the lab’s microscope to apply no more power to the tissue than a cat toy laser pointer (less than 5 milliwatts).
“You should make sure you are not changing or affecting the neuronal physiology in any adverse way,” Yildirim said. “You should really keep everything intact and make sure you are not bringing something external that could be damaging. That’s why we are so careful about power (and chemical labeling).”
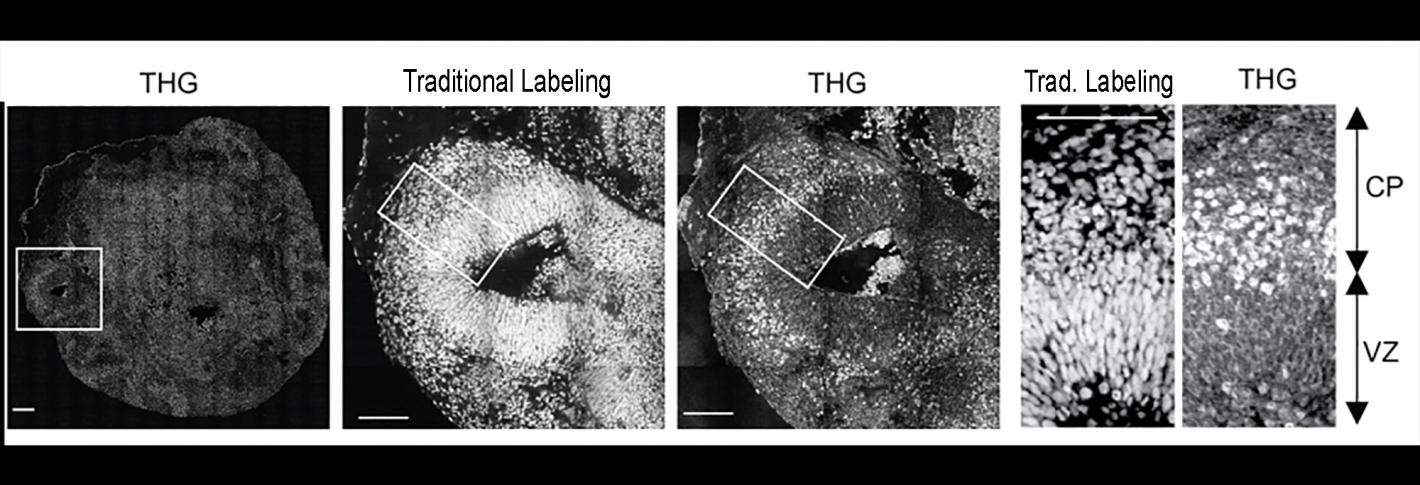
Above: Without the use of any chemical labels third harmonic generation (THG) microscopy performs well compared to techniques using traditional labeling methods.. It allowed for distinguishing the ventricular zone (VZ) from the cortical plate (CP).
Even at low power, they achieved adequate signal to achieve label-free, intact imaging of fixed and live organoids. To validate that they compared their THG images to images made via more traditional chemical labeling methods.
The THG system allowed them to track the migration of newborn neurons as they made their way from the rim around open spaces in the minibrains (called ventricles) to the outer edge, which is directly analogous to the brain’s cortex. They saw that the nascent neurons in the minibrains modeling Rett syndrome moved slowly and in meandering paths compared to the faster motion in straighter lines exhibited by the same cell types in minibrains without MECP2 mutation. Sur said the consequences of such migration deficits are consistent with what scientists, including in his lab, have hypothesized is going on in Rett syndrome fetuses.
“We know from postmortem brains and brain imaging methods that things go awry during brain development in Rett syndrome, but it has been astonishingly difficult to figure out what and why,” said Sur, who directs the Simons Center for the Social Brain at MIT.“This method has enabled us to directly visualize a key contributor.” THG images tissues without labels because it is very sensitive to changes in the refractive index of materials, Yildirim said. It therefore resolves boundaries between biological structures, such as blood vessels, cell membranes and extracellular spaces. Because neural shapes change during their development, the team was able to also clearly see the delineation between the ventricular zone (the area around the ventricles where the newborn neurons emerge) and the cortical plate (an area that mature neurons settle into). It was also very easy to resolve various ventricles and segment them into distinct regions.
Those properties allowed the researchers to be able to see that in Rett syndrome organoids the ventricles were larger and more numerous and that the ventricular zones—the rims around the ventricles where neurons are born—were thinner. In live organoids they were able to track some of the neurons making their way toward the cortex over a few days, taking a new picture every 20 minutes, as neurons in real developing brains also attempt to do. They saw that Rett syndrome neurons achieved only about two thirds the speed of non-mutated neurons. The paths of the Rett neurons were also significantly more wiggly. The two differences combined meant that the Rett cells barely got half as far.
“We now want to know how MECP2 influences genes and molecules that influence neuronal migration,” Sur said. “By screening Rett syndrome organoids, we have some good guesses, which we are eager to test.” Yildirim, who will launch his own lab as an assistant professor at the Cleveland Clinic’s Lerner Research Institute in September, said he has new questions based on the findings. He wants to image later in organoid development to track the consequences of the sinuous migration. He also wants to find out more about whether specific cell types struggle to migrate more or less, which could alter how cortical circuits work.
Yildirim also said he hopes to continue advancing THG three-photon microscopy, which he sees as having potential for fine-grained imaging in humans. It can be an important advantage in people especially that the imaging method can penetrate deep into living tissue without the need for artificial labels.
In addition to Yildirim, Sur and So, the paper’s other authors are Chloe Delepine, Danielle Feldman, Vincent Pham, Stephanie Chou, Jacque Pak Kan Ip, Alexi Nott, Li-Huei Tsai, and Guo-li Ming.
The National Institutes of Health, The National Science Foundation, the JPB Foundation and the Massachusetts Life Sciences Initiative provided funding for the research.




