Before Picower Professor Li-Huei Tsai came to MIT in 2006 she had discovered that misregulation of the enzyme Cdk5 produced a powerful mouse model of Alzheimer’s symptoms including neurodegeneration and memory loss. At The Picower Institute her lab used these mice to investigate potential restorative interventions, including ways to increase the ability of remaining neurons to make and sustain circuit connections needed to recall memories. The lab found that Alzheimer’s model mice in more stimulating and enriching environments (e.g. with more toys) showed better memory performance than mice in normal cages, suggesting that environmental factors affecting gene expression (“epigenetic” factors), might be implicated.
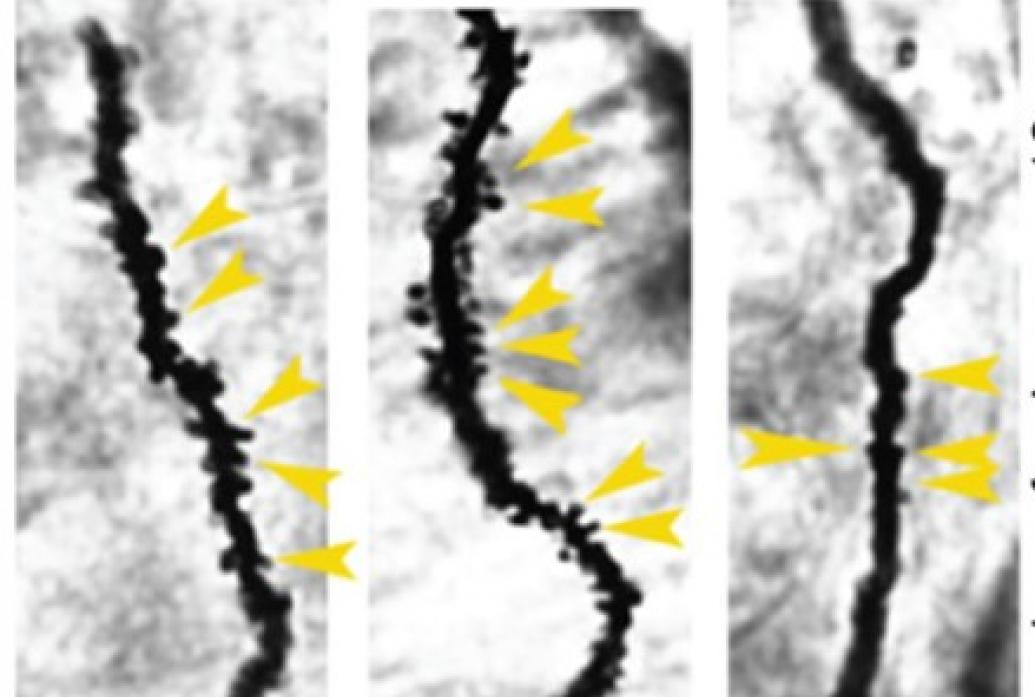
In a landmark paper in 2007, the lab identified a molecular mechanism when they inhibit HDAC enzymes that affect how chromatin physically locks down the expression of genes needed for promoting neural “plasticity,” the property needed to form new circuit connections. Alzheimer’s mice in which they inhibited HDACs were able to recall memories that untreated mice could not. In 2009 her group identified HDAC2 as the specific HDAC enzyme that regulated neuronal expression of genes supporting synaptic plasticity, learning, and memory. HDAC2 was the target of the effective inhibitors.
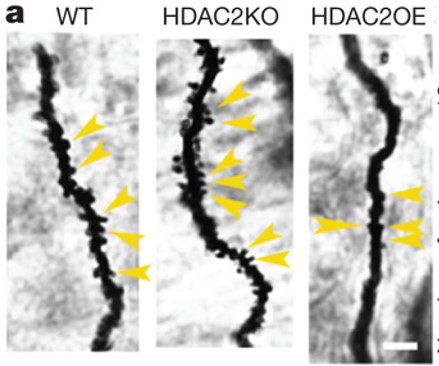
The phenomenon was not an artifact of modeling Alzheimer’s in mice. In 2012 the lab showed that HDAC2 is markedly increased in hippocampal neurons of postmortem human AD patient brains, confirming that an epigenetic blockade of neuronal gene expression under disease conditions contributes to the synaptic and memory deficits observed in human neurodegeneration. To further advance the discovery as a therapeutic strategy, in 2017 the lab showed that deactivating an HDAC2 transcription factor, Sp3, in AD-model mice had similar effects as inhibiting HDAC2 for restoring memory. This provided a new drug target that pharmaceutical companies have since been investigating.

