Diseases such as Alzheimer’s, Parkinson’s, ALS and Huntington’s are “neurodegenerative” because over time more and more neurons die. A critical question in the study of these diseases is what factors contribute to their demise and what factors could forestall it. Associate Professor Myriam Heiman has developed innovative techniques to help discover such factors.
In 2015, after identifying a potential molecular pathway that appeared to contribute to the death of specific brain cells in people with the mutation that causes Huntington’s disease, Heiman’s lab devised a novel way, called “SLIC,” of probing which specific genes in the broader pathway might “cooperate” with the mutation to cause toxicity. The lab interfered with 28 candidate genes, one by one, in the targeted neurons in HD-model and healthy control mice to see the effect. They found that knocking down a specific gene, Gpx6, enhanced toxicity in the cells of HD-model mice and that overexpressing the gene reduced toxicity.
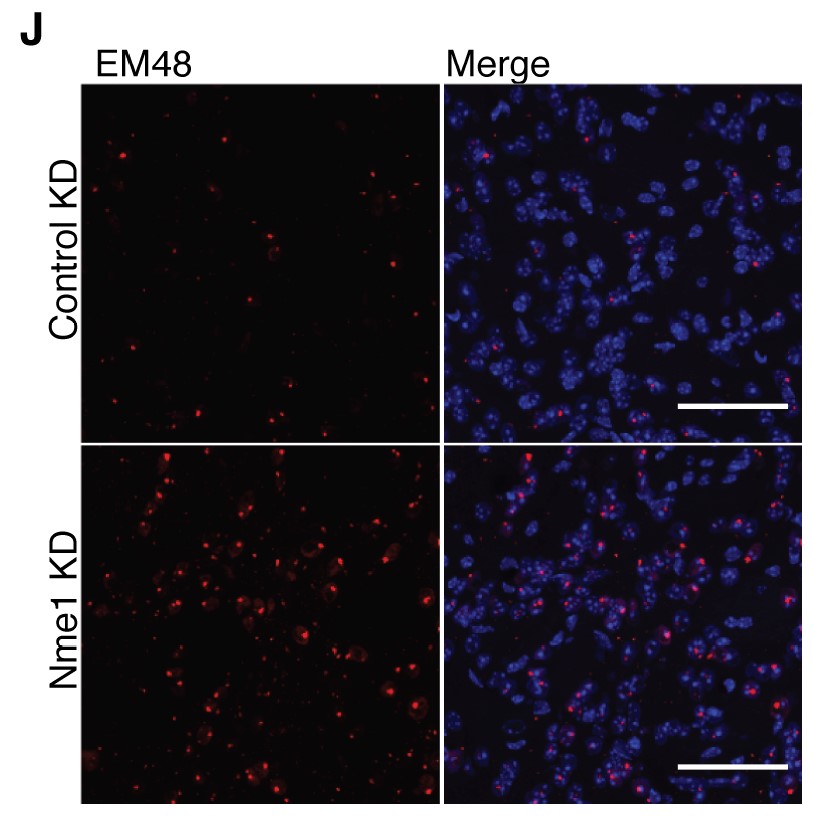
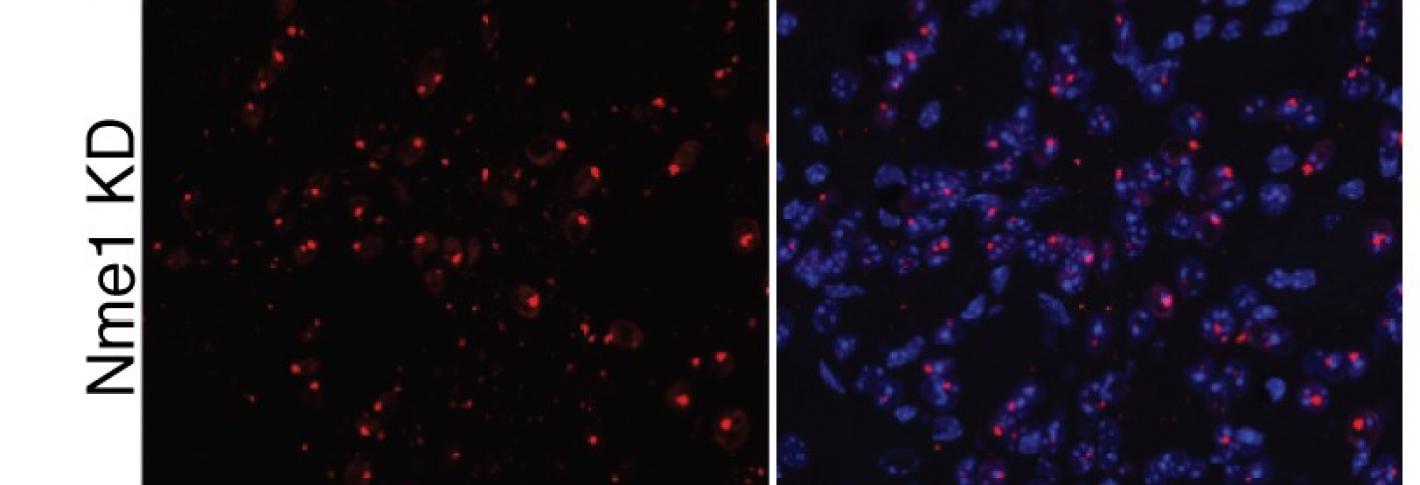
In 2020 the lab advanced the idea much further, accomplishing the first genome-wide screening in a mammalian brain for the genes necessary for the survival of neurons in a region called the striatum. The unbiased method allowed them to assess the effects of knocking down each of the 22,000 genes expressed in the mouse brain, one by one in cells. The study enabled the discovery of many genes that had not been known before to be vital to neuronal survival. The team also performed the screen in HD-model mice and found that the Nme family of genes, which regulate others responsible for proper disposal of proteins, were especially important for sustaining cells in that disease context. They showed that when they overexpressed Nme in cells in HD mice, they survived better.
The lab hopes to apply this technique to help identify factors that contribute to neural vulnerability in many other brain disease contexts, including Parkinson’s disease.

