The brain contains multitudes. Just the category of “neurons” comprises hundreds, if not thousands, of different types of nerve cells and the brain has many other kinds of non-neuronal cells, too, including in the blood vessels that sustain the organ. Any one of these cell types can turn out to be more or less vulnerable to a particular brain disease. Understanding how disease emerges and what to do to stop it often requires learning which kinds of cells are most susceptible, since these cells’ demise often starts a cascade of negative effects in the brain. In her disease-focused research, Associate Professor Myriam Heiman has used cutting edge techniques, including one she helped invent as a postdoc, to understand how specific types of cells become vulnerable in disease.
The vast variety of brain cells all share the same DNA. What makes them differ, under normal and disease circumstances, is how they express those instructions by transcribing select sequences of DNA (i.e. genes) into mRNA and then translating that mRNA into proteins in cellular structures called ribosomes. To understand how cells differ and how they are affected by disease, Heiman therefore focuses on measuring RNA transcription and translation in different cell types and then testing the effects of the differences she observes by experimentally manipulating expression of the genes in mice.
In 2020 Heiman’s lab performed the first study to comprehensively track how different subtypes of brain cells respond to the mutation that causes Huntington’s disease (HD). Using two different screening methods, “TRAP,” used to measure translation in HD model mice, and single-cell RNA sequencing, which measures transcription in HD mice and postmortem HD human brains, the team discovered that a significant cause of death for an especially afflicted kind of cell, the spiny projection neuron (SPN), is an immune response to genetic material errantly released by mitochondria. The study also highlighted a potential role for “Rarb,” or retinoic acid receptor b, a master transcriptional regulator of many important genes whose expression differs in HD.
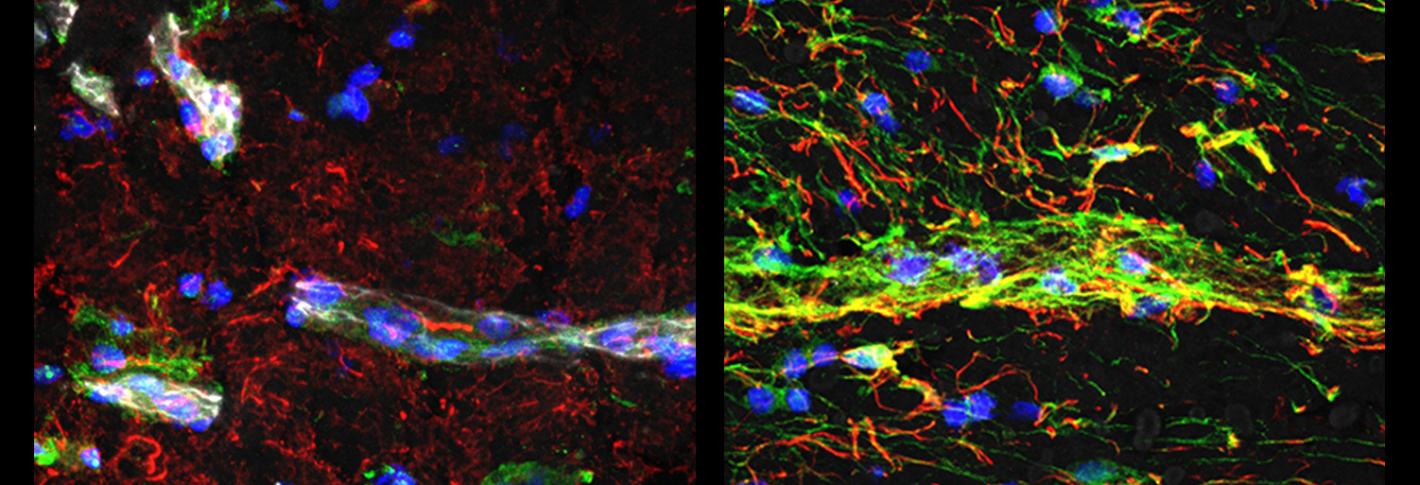
Above: In vessels of people with Huntington's disease (right image) researchers saw much higher indications of innate immune signaling (green) and much lower levels of a protein associated with blood-brain barrier integrity (white), than in people who did not have the disease (left image). Images by Francisco Garcia.
In 2022, Heiman’s lab co-led another pioneering study using single-cell RNA sequencing to comprehensively characterize 11 different cell types of the brain’s blood vessels, both in health and amid neurodegenerative disease. One key finding is that certain vascular cells express genes differently depending on where they are located. They also found multiple gene expression differences in vascular cells of people with HD vs. people without the condition that likely undermine the integrity of the blood-brain barrier, a crucial means of regulating what goes into or comes out of the brain.

