About 25 percent of people have the APOE4 variant of the APOE gene, which puts them at substantially greater risk for Alzheimer’s disease. Almost everyone with Alzheimer’s, and even some elderly people without, suffer from cerebral amyloid angiopathy (CAA), a condition in which amyloid protein deposits on blood vessel walls impair the ability of the blood-brain barrier (BBB) to properly transport nutrients, clear out waste and prevent the invasion of pathogens and unwanted substances.
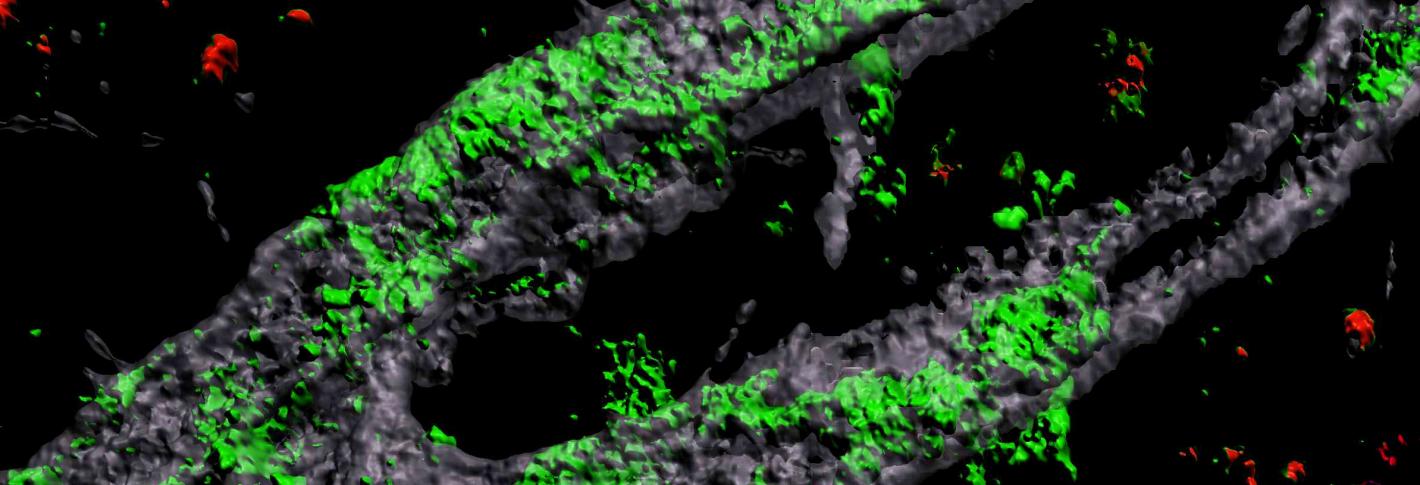
Above: A 3D rendering of an APOE4 carrying engineered blood vessel shows heavy accumulation of amyloid protein (green).
To investigate the connection between Alzheimer’s, the APOE4 variant and CAA, Joel Blanchard, a postdoc in the lab of Li-Huei Tsai, and co-authors coaxed human induced pluripotent stem cells to become the three types of cells that make up the BBB: brain endothelial cells, astrocytes and pericytes. Pericytes were modeled by mural cells that they tested extensively to ensure they exhibited pericyte-like properties and gene expression.
Grown for two weeks within a three-dimensional hydrogel scaffold, the lab-engineered human blood-brain barrier model’s cells assembled into vessels that exhibited natural BBB properties, including low permeability to molecules and expression of the same key genes, proteins and molecular pumps as natural BBBs. When immersed in culture media high in amyloid proteins, mimicking conditions in Alzheimer’s disease brains, the lab-grown BBB models exhibited the same kind of amyloid accumulation seen in human disease.
With a model BBB established, they then sought to test the difference APOE4 makes. They showed by several measures that APOE4-carrying BBB models accumulated more amyloid from culture media than those carrying APOE3, the more typical and healthy variant.
To pinpoint how APOE4 makes that difference, they engineered eight different versions covering all the possible combinations of the three cell types having either APOE3 or APOE4. When exposed these month-old models to amyloid-rich media, only versions with APOE4 pericyte-like mural cells showed excessive accumulation of amyloid proteins. Replacing APOE4 mural cells with APOE3-carrying ones reduced amyloid deposition. These results, validated in mice and human tissue and published in Nature Medicine in 2020, put blame for CAA-like pathology squarely on APOE4-carrying pericytes. The study went on to identify the molecular pathway responsible and how medicines may treat it.

